What Are Three Functions That Lipids Serve In Plants And/or Animals?
Biological Macromolecules
11 Lipids
Learning Objectives
By the end of this section, you will exist able to do the following:
- Draw the four major types of lipids
- Explicate the role of fats in storing energy
- Differentiate between saturated and unsaturated fat acids
- Describe phospholipids and their role in cells
- Ascertain the basic structure of a steroid and some steroid functions
- Explicate how cholesterol helps maintain the plasma membrane's fluid nature
Lipids include a various group of compounds that are largely nonpolar in nature. This is considering they are hydrocarbons that include more often than not nonpolar carbon–carbon or carbon–hydrogen bonds. Not-polar molecules are hydrophobic ("water fearing"), or insoluble in h2o. Lipids perform many unlike functions in a jail cell. Cells store free energy for long-term use in the form of fats. Lipids besides provide insulation from the environment for plants and animals ((Figure)). For example, they help go along aquatic birds and mammals dry when forming a protective layer over fur or feathers because of their water-repellant hydrophobic nature. Lipids are also the building blocks of many hormones and are an important constituent of all cellular membranes. Lipids include fats, oils, waxes, phospholipids, and steroids.
Hydrophobic lipids in aquatic mammals' fur, such every bit this river otter, protect them from the elements. (credit: Ken Bosma)

Fats and Oils
A fat molecule consists of two chief components—glycerol and fat acids. Glycerol is an organic compound (alcohol) with three carbons, 5 hydrogens, and three hydroxyl (OH) groups. Fat acids have a long chain of hydrocarbons to which a carboxyl group is attached, hence the name "fat acid." The number of carbons in the fatty acid may range from 4 to 36. The virtually common are those containing 12–18 carbons. In a fatty molecule, the fatty acids attach to each of the glycerol molecule's three carbons with an ester bond through an oxygen atom ((Figure)).
Joining three fat acids to a glycerol backbone in a dehydration reaction forms triacylglycerol. 3 water molecules release in the procedure.
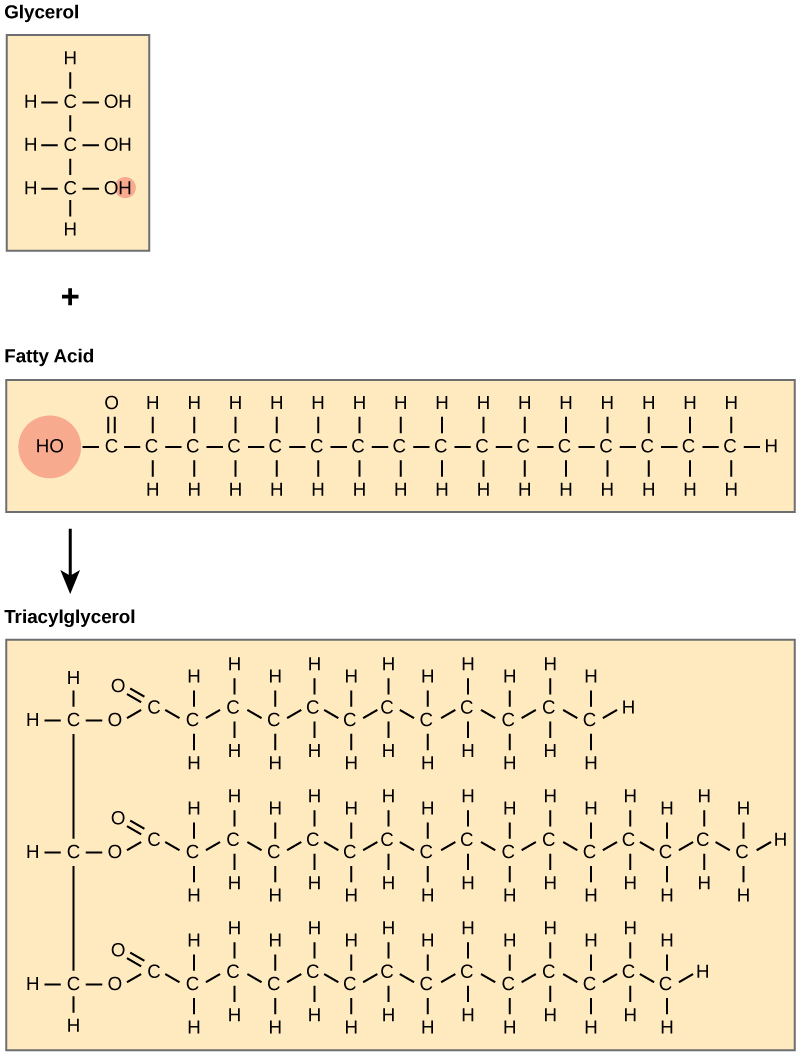
During this ester bond germination, 3 water molecules are released. The three fatty acids in the triacylglycerol may be similar or different. We also call fats triacylglycerols or triglycerides because of their chemical construction. Some fatty acids take common names that specify their origin. For instance, palmitic acid, a saturated fat acid, is derived from the palm tree. Arachidic acid is derived from Arachis hypogea, the scientific proper noun for groundnuts or peanuts.
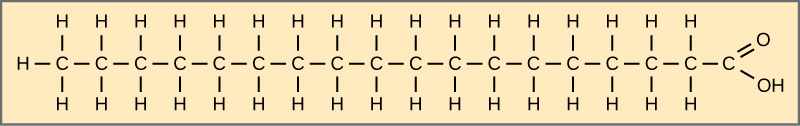
Fatty acids may be saturated or unsaturated. In a fatty acid chain, if there are simply single bonds betwixt neighboring carbons in the hydrocarbon chain, the fatty acid is saturated. Saturated fatty acids are saturated with hydrogen. In other words, the number of hydrogen atoms attached to the carbon skeleton is maximized. Stearic acrid is an case of a saturated fatty acid ((Figure)).
Stearic acid is a common saturated fatty acid.
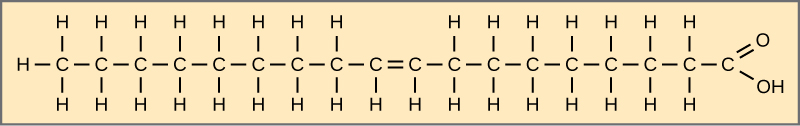
When the hydrocarbon concatenation contains a double bond, the fat acid is unsaturated. Oleic acid is an example of an unsaturated fatty acid ((Figure)).
Oleic acid is a mutual unsaturated fatty acid.
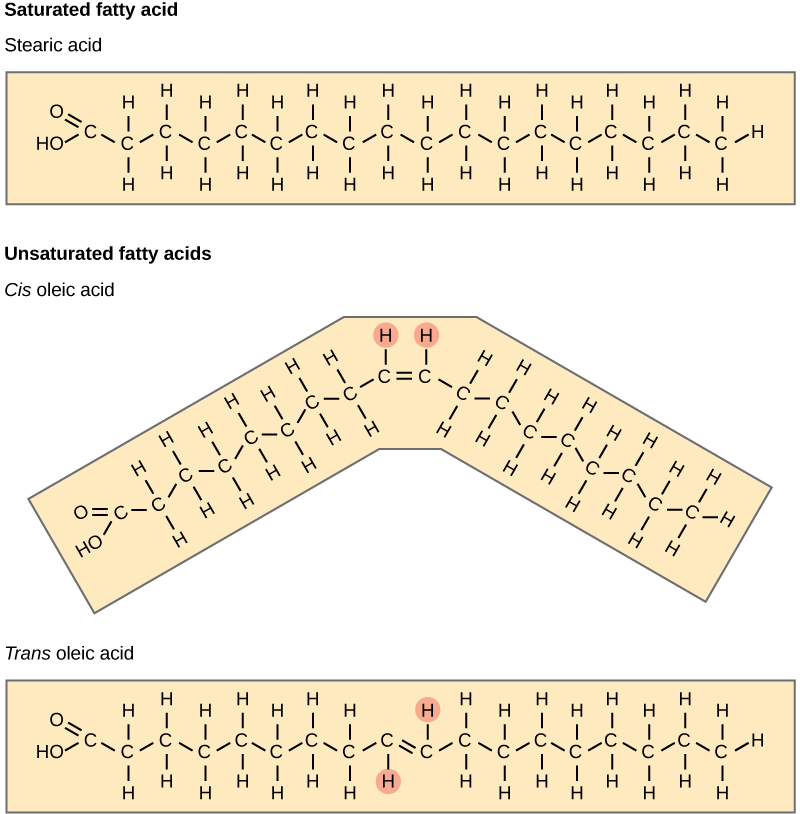
Most unsaturated fats are liquid at room temperature. We call these oils. If there is one double bond in the molecule, then it is a monounsaturated fat (due east.thousand., olive oil), and if there is more one double bail, and so it is a polyunsaturated fat (due east.g., canola oil).
When a fatty acid has no double bonds, information technology is a saturated fatty acrid considering it is not possible to add more than hydrogen to the chain'south carbon atoms. A fat may contain similar or dissimilar fatty acids attached to glycerol. Long straight fatty acids with single bonds by and large pack tightly and are solid at room temperature. Brute fats with stearic acid and palmitic acid (mutual in meat) and the fat with butyric acrid (common in butter) are examples of saturated fats. Mammals store fats in specialized cells, or adipocytes, where fat globules occupy most of the cell's volume. Plants store fat or oil in many seeds and use them as a source of energy during seedling development. Unsaturated fats or oils are ordinarily of plant origin and comprise cis unsaturated fatty acids. Cis and trans indicate the configuration of the molecule effectually the double bond. If hydrogens are present in the same aeroplane, it is a cis fat. If the hydrogen atoms are on two different planes, it is a trans fat. The cis double bond causes a curve or a "kink" that prevents the fatty acids from packing tightly, keeping them liquid at room temperature ((Figure)). Olive oil, corn oil, canola oil, and cod liver oil are examples of unsaturated fats. Unsaturated fats aid to lower blood cholesterol levels; whereas, saturated fats contribute to plaque formation in the arteries.
Saturated fat acids have hydrocarbon chains continued by single bonds merely. Unsaturated fatty acids have one or more double bonds. Each double bond may be in a cis or trans configuration. In the cis configuration, both hydrogens are on the same side of the hydrocarbon chain. In the trans configuration, the hydrogens are on contrary sides. A cis double bond causes a kink in the chain.
Trans Fats
The food industry artificially hydrogenates oils to brand them semi-solid and of a consistency desirable for many processed nutrient products. Simply speaking, hydrogen gas is bubbled through oils to solidify them. During this hydrogenation process, double bonds of the cis– conformation in the hydrocarbon chain may convert to double bonds in the trans– conformation.
Margarine, some types of peanut butter, and shortening are examples of artificially hydrogenated trans fats. Recent studies take shown that an increment in trans fats in the homo diet may lead to college levels of low-density lipoproteins (LDL), or "bad" cholesterol, which in turn may lead to plaque deposition in the arteries, resulting in center disease. Many fast nutrient restaurants take recently banned using trans fats, and food labels are required to display the trans fat content.
Omega Fatty Acids
Essential fatty acids are those that the human body requires only does not synthesize. Consequently, they have to be supplemented through ingestion via the nutrition. Omega-iii fatty acids (similar those in (Figure)) fall into this category and are one of only two known for humans (the other is omega-6 fatty acid). These are polyunsaturated fatty acids and are omega-3 because a double bond connects the third carbon from the hydrocarbon chain's end to its neighboring carbon.
Alpha-linolenic acrid is an example of an omega-iii fat acid. It has iii cis double bonds and, as a event, a curved shape. For clarity, the diagram does not testify the carbons. Each singly bonded carbon has two hydrogens associated with it, which the diagram as well does not show.
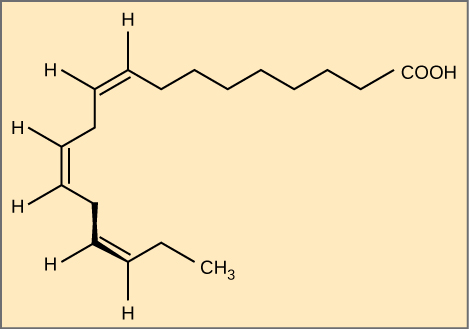
The farthest carbon abroad from the carboxyl group is numbered equally the omega (ω) carbon, and if the double bond is betwixt the third and quaternary carbon from that stop, it is an omega-3 fatty acid. Nutritionally important because the body does not make them, omega-3 fat acids include blastoff-linoleic acid (ALA), eicosapentaenoic acid (EPA), and docosahexaenoic acrid (DHA), all of which are polyunsaturated. Salmon, trout, and tuna are good sources of omega-3 fatty acids. Enquiry indicates that omega-3 fatty acids reduce the risk of sudden death from center attacks, lower triglycerides in the blood, decrease claret pressure, and forestall thrombosis by inhibiting blood clotting. They also reduce inflammation, and may assistance lower the adventure of some cancers in animals.
Similar carbohydrates, fats take received considerable bad publicity. It is true that eating an excess of fried foods and other "fatty" foods leads to weight gain. However, fats do accept important functions. Many vitamins are fatty soluble, and fats serve as a long-term storage form of fatty acids: a source of free energy. They also provide insulation for the body. Therefore, nosotros should consume "good for you" fats in moderate amounts on a regular basis.
Waxes
Wax covers some aquatic birds' feathers and some plants' leaf surfaces. Considering of waxes' hydrophobic nature, they prevent water from sticking on the surface ((Effigy)). Long fatty acrid chains esterified to long-concatenation alcohols incorporate waxes.
Lipids comprise waxy coverings on some leaves. (credit: Roger Griffith)

Phospholipids
Phospholipids are major plasma membrane constituents that contain cells' outermost layer. Like fats, they are comprised of fatty acid bondage attached to a glycerol or sphingosine courage. However, instead of 3 fat acids attached equally in triglycerides, there are two fatty acids forming diacylglycerol, and a modified phosphate group occupies the glycerol backbone'south third carbon ((Figure)). A phosphate group lone attached to a diaglycerol does not authorize every bit a phospholipid. Information technology is phosphatidate (diacylglycerol 3-phosphate), the precursor of phospholipids. An alcohol modifies the phosphate group. Phosphatidylcholine and phosphatidylserine are two important phospholipids that are in plasma membranes.
A phospholipid is a molecule with two fatty acids and a modified phosphate grouping attached to a glycerol backbone. Calculation a charged or polar chemical grouping may modify the phosphate.
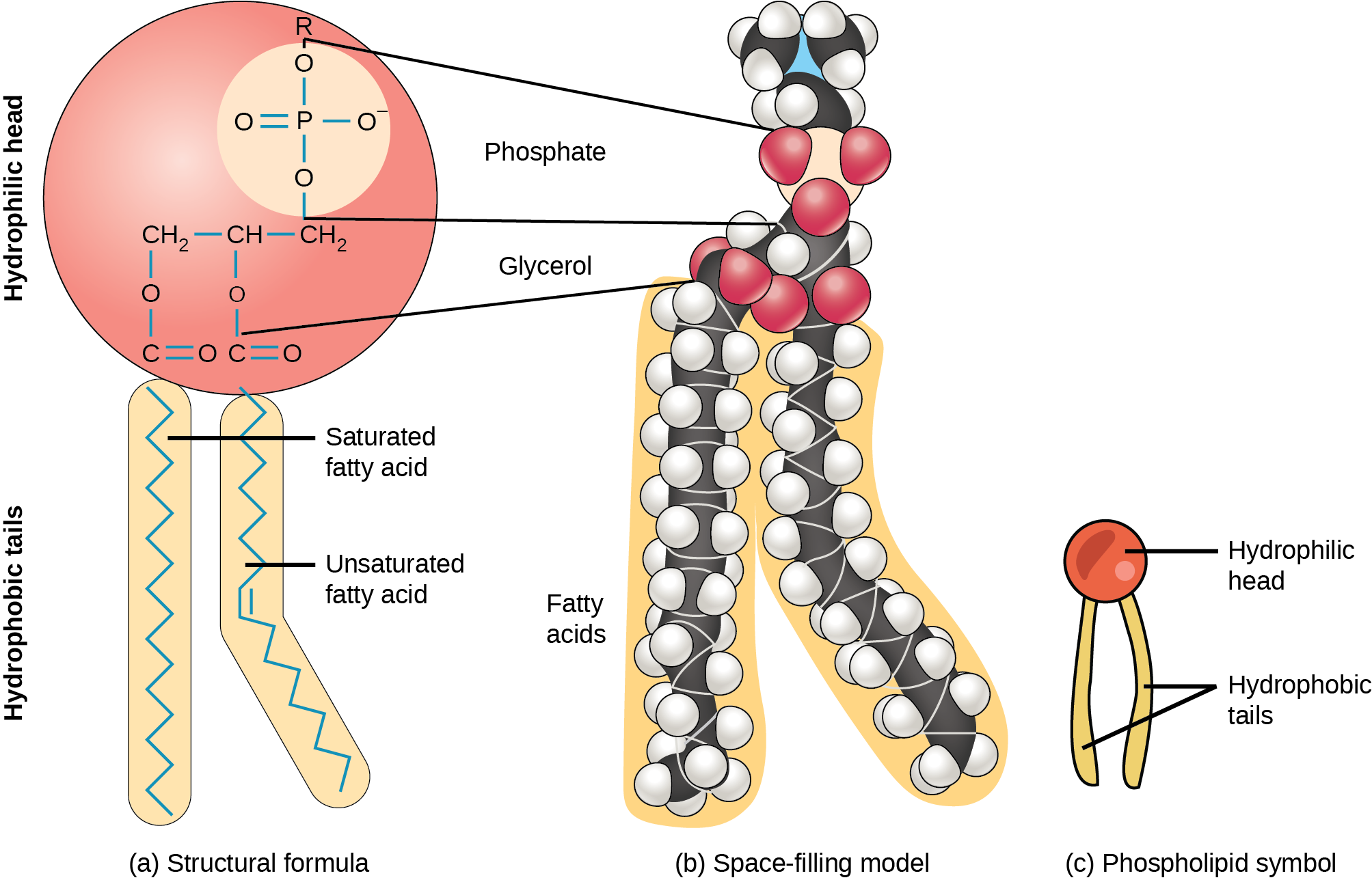
A phospholipid is an amphipathic molecule, significant it has a hydrophobic and a hydrophilic part. The fatty acrid bondage are hydrophobic and cannot interact with h2o; whereas, the phosphate-containing group is hydrophilic and interacts with water ((Figure)).
The phospholipid bilayer is the major component of all cellular membranes. The hydrophilic head groups of the phospholipids face the aqueous solution. The hydrophobic tails are sequestered in the center of the bilayer.
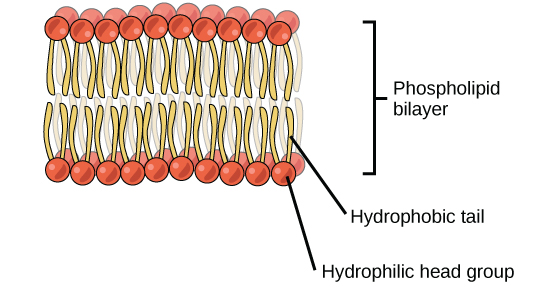
The head is the hydrophilic office, and the tail contains the hydrophobic fatty acids. In a membrane, a bilayer of phospholipids forms the structure's matrix, phospholipids' fatty acrid tails face within, abroad from water; whereas, the phosphate grouping faces the outside, aqueous side ((Effigy)).
Phospholipids are responsible for the plasma membrane's dynamic nature. If a drop of phospholipids is placed in h2o, information technology spontaneously forms a structure that scientists telephone call a micelle, where the hydrophilic phosphate heads face up the outside and the fatty acids face up the construction'south interior.
Steroids
Unlike the phospholipids and fats that we discussed earlier, steroids have a fused ring structure. Although they do not resemble the other lipids, scientists group them with them because they are too hydrophobic and insoluble in water. All steroids have four linked carbon rings and several of them, like cholesterol, have a short tail ((Figure)). Many steroids too take the –OH functional group, which puts them in the alcohol classification (sterols).
Four fused hydrocarbon rings incorporate steroids such as cholesterol and cortisol.
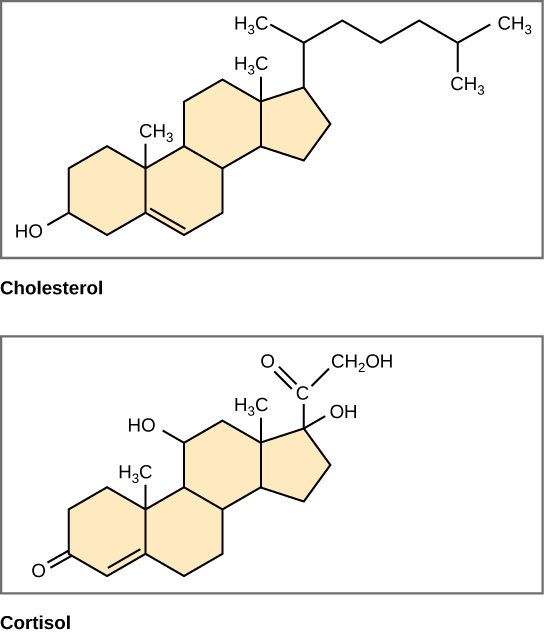
Cholesterol is the most common steroid. The liver synthesizes cholesterol and is the precursor to many steroid hormones such as testosterone and estradiol, which gonads and endocrine glands secrete. Information technology is also the forerunner to Vitamin D. Cholesterol is as well the precursor of bile salts, which help emulsifying fats and their subsequent absorption past cells. Although lay people often speak negatively about cholesterol, information technology is necessary for the torso's proper performance. Sterols (cholesterol in animal cells, phytosterol in plants) are components of the plasma membrane of cells and are found inside the phospholipid bilayer.
Section Summary
Lipids are a class of macromolecules that are nonpolar and hydrophobic in nature. Major types include fats and oils, waxes, phospholipids, and steroids. Fats are a stored form of free energy and are as well known every bit triacylglycerols or triglycerides. Fats are comprised of fat acids and either glycerol or sphingosine. Fat acids may be unsaturated or saturated, depending on the presence or absence of double bonds in the hydrocarbon chain. If only single bonds are present, they are saturated fatty acids. Unsaturated fatty acids may have one or more than double bonds in the hydrocarbon chain. Phospholipids comprise the membrane's matrix. They accept a glycerol or sphingosine courage to which two fatty acrid chains and a phosphate-containing group are attached. Steroids are another class of lipids. Their basic structure has four fused carbon rings. Cholesterol is a type of steroid and is an important constituent of the plasma membrane, where it helps to maintain the membrane's fluid nature. It is too the precursor of steroid hormones such as testosterone.
Review Questions
Saturated fats take all of the following characteristics except:
- they are solid at room temperature
- they accept single bonds within the carbon chain
- they are unremarkably obtained from animal sources
- they tend to deliquesce in water easily
D
Phospholipids are important components of ________.
- the plasma membrane of cells
- the ring structure of steroids
- the waxy roofing on leaves
- the double bond in hydrocarbon bondage
A
Cholesterol is an integral function of plasma membranes. Based on its structure, where is it found in the membrane?
- on the extracellular surface
- embedded with the phospholipid heads
- within the tail bilayer
- attached to the intracellular surface
C
Critical Thinking Questions
Explain at to the lowest degree three functions that lipids serve in plants and/or animals.
Fatty serves as a valuable mode for animals to store energy. It can also provide insulation. Waxes can protect plant leaves and mammalian fur from getting wet. Phospholipids and steroids are important components of animal jail cell membranes, every bit well as establish, fungal, and bacterial membranes.
Why have trans fats been banned from some restaurants? How are they created?
Trans fats are created artificially when hydrogen gas is bubbled through oils to solidify them. The double bonds of the cis conformation in the hydrocarbon chain may be converted to double bonds in the trans configuration. Some restaurants are banning trans fats because they crusade higher levels of LDL, or "bad"cholesterol.
Why are fatty acids improve than glycogen for storing big amounts of chemical free energy?
Fats have a higher energy density than carbohydrates (averaging 9kcal/gram versus 4.3kcal/gram respectively). Thus, on a per gram basis, more free energy can exist stored in fats than can be stored in carbohydrates. Additionally, fats are packaged into spherical globules to minimize interactions with the water-based plasma membrane, while glycogen is a big branched carbohydrate that cannot be compacted for storage.
Part of cortisol'south office in the body involves passing through the plasma membrane to initiate signaling within a prison cell. Describe how the structures of cortisol and the plasma membrane allow this to occur.
Cortisol is a small, by and large hydrophobic molecule, while the phospholipids that create plasma membranes accept a hydrophilic caput and hydrophobic tails. Since cortisol is hydrophobic, it can interact with the sequestered tails of the phospholipids in the middle of the plasma membrane. This, along with its minor size, allows cortisol to move through the plasma membrane to the within of the cell.
Glossary
- lipid
- macromolecule that is nonpolar and insoluble in water
- omega fat
- type of polyunsaturated fat that the body requires; numbering the carbon omega starts from the methyl terminate or the cease that is uttermost from the carboxylic end
- phospholipid
- membranes' major constituent; comprised of two fatty acids and a phosphate-containing grouping fastened to a glycerol courage
- saturated fatty acid
- long-chain hydrocarbon with unmarried covalent bonds in the carbon chain; the number of hydrogen atoms attached to the carbon skeleton is maximized
- steroid
- type of lipid comprised of four fused hydrocarbon rings forming a planar construction
- trans fat
- fat formed artificially by hydrogenating oils, leading to a different arrangement of double bail(south) than those in naturally occurring lipids
- triacylglycerol (also, triglyceride)
- fat molecule; consists of three fatty acids linked to a glycerol molecule
- unsaturated fatty acid
- long-chain hydrocarbon that has ane or more double bonds in the hydrocarbon concatenation
- wax
- lipid comprised of a long-chain fatty acrid that is esterified to a long-chain booze; serves as a protective coating on some feathers, aquatic mammal fur, and leaves
Source: https://opentextbc.ca/biology2eopenstax/chapter/lipids/
Posted by: hallwhats1993.blogspot.com

0 Response to "What Are Three Functions That Lipids Serve In Plants And/or Animals?"
Post a Comment