Which Animal Has A Vagina Most Like A Human
- Review
- Open Access
- Published:
A review of the human vs. porcine female person genital tract and associated allowed system in the perspective of using minipigs every bit a model of human genital Chlamydia infection
Veterinarian Research volume 46, Article number:116 (2015) Cite this commodity
Abstruse
Sexually transmitted diseases establish major wellness issues and their prevention and treatment continue to challenge the wellness care systems worldwide. Brute models are essential for a deeper understanding of the diseases and the development of safe and protective vaccines. Currently a good predictive not-rodent model is needed for the report of genital chlamydia in women. The pig has get an increasingly popular model for homo diseases due to its close similarities to humans. The aim of this review is to compare the porcine and man female person genital tract and associated allowed system in the perspective of genital Chlamydia infection. The comparison of women and sows has shown that despite some gross anatomical differences, the structures and proportion of layers undergoing cyclic alterations are very similar. Reproductive hormonal cycles are closely related, only showing a slight departure in cycle length and source of luteolysing hormone. The epithelium and functional layers of the endometrium show similar cyclic changes. The immune arrangement in pigs is very similar to that of humans, fifty-fifty though pigs have a higher percent of CD4+/CD8+ double positive T cells. The genital immune system is likewise very like in terms of the circadian fluctuations in the mucosal antibody levels, but differs slightly regarding immune cell infiltration in the genital mucosa - predominantly due to the influx of neutrophils in the porcine endometrium during rut. The vaginal flora in Göttingen Minipigs is non dominated by lactobacilli as in humans. The vaginal pH is effectually 7 in Göttingen Minipigs, compared to the more acidic vaginal pH effectually 3.5–v in women. This review reveals important similarities between the human and porcine female person reproductive tracts and proposes the pig every bit an advantageous supplementary model of human genital Chlamydia infection.
Tabular array of contents
1. Introduction
2. Methods
iii. The female reproductive cycles
4. The female genital tract in pigs and humans
4.1 Gross anatomy
four.2 Microscopic anatomy
four.2.one Vagina
4.two.2 Neck
four.2.3 Uterus
four.2.4 Fallopian tubes
4.three Anatomical and histological differences of relevance for a Chlamydia model
5. Genetics
vi. The porcine immune organization compared to the human being immune system
vi.ane The genital mucosal immune arrangement
half dozen.1.ane Distribution of allowed cells in the genital tract tissue
6.1.2 The humoral genital allowed response
half dozen.two Immunological differences of relevance for a Chlamydia model
7. The vaginal flora and pH
8. Important differences between rodents and minipigs
9. Conclusions
ten. List of abbreviations
11. Competing interests
12. Authors' contributions
13. Authors' information
14. References
ane. Introduction
Brute models are essential for gaining new insight into disease mechanisms of homo genital diseases and the development of new prophylactic strategies and treatments [1]. Predominantly rodents are used equally models, within pre-clinical enquiry, with mice often being the brute of choice [2,3]. Rodent models have articulate advantages both regarding applied problems, by being small and easy to handle, and economically affordable [2]. Furthermore, several genetically modified knockout strains are easily accessible, creating a unique opportunity to study the role of specific mediators in the immune response [4,5].
However, when evaluating animal models, dissimilar parameters are of import to consider depending on the purpose of the model [half dozen]:
-
Face validity; how well is the biology and symptoms of the human affliction mimicked by the model.
-
Predictive validity; how well is the effect of a drug/compound or treatment mimicked past the model.
-
Target validity; how similar a role the target of interest plays in the model compared to humans.
Despite the many advantages of rodent models, rodents show a number of differences to humans in terms of size, beefcake, physiology and immunology that exercise not e'er let them to mimic the human being form of infection and allowed response [4,5,seven,8]. The face validity and predictive validity is therefore decumbent to be insufficient, leaving a potent need for an intermediate and reliable model for the study of female genital tract (FGT) infections and the development of appropriate vaccines confronting them [9,ten]. Not-human primates (NHP) are the animals most closely related to humans and therefore likely to evidence the greatest face- and predictive validity. However, due to ethical concerns and costly experiments associated with studies in NHP, in that location is a need for an intermediate pre-clinical/advanced not-rodent animal model.
The pig has get an increasingly popular model, especially within the fields of atherosclerosis and diabetes research, because of its physiological and anatomical similarities to humans [11-13]. Pigs of reduced body size such as the Göttingen Minipigs offer a great advantage by having a smaller size at sexual maturity and a lower growth rate than conventional pigs [14]. Furthermore, such breeds are available as specific pathogen costless from specialized convenance companies [15]. Wherever possible, this review will focus on the minipig, since this has been the experimental animal of choice in our inquiry. Despite the physical size, there are no studies reporting whatever physiological differences betwixt minipigs and conventional pigs. Furthermore, Göttingen Minipigs are partly derived from High german Landrace pigs [xv].
Information technology has recently been shown that pigs are susceptible to Chlamydia trachomatis, the amanuensis causing human genital Chlamydia, and that pigs are suitable models for the study of Chlamydia pathogenesis and evaluation of vaccine candidates [xvi]. To evaluate the pig equally a model of human genital Chlamydia and to be able to interpret and extrapolate results critically and reliably, it is of import to understand the morphological and functional similarities and differences between the human and porcine female reproductive systems. The purpose of this review is to provide the basis for this agreement.
ii. Methods
The PubMed database [17], Google Scholar [18] and CAB ABSTRACTS database were searched, with the post-obit keywords: Pig/swine/porcine, genital tract/reproductive tract/vagina/cervix/uterus/uterine body/uterine horn/Fallopian tubes, immunology/immune response/immunity, mucosal immunity/immune response, estrous cycle/menstrual cycle/ sex hormone regulation immunity, grunter model/porcine model/brute model, sexually transmitted disease/genital infections, vaginal microbiota/flora/ecosystem.
Due to the very limited numbers of original published papers inside the search criteria no year limit was applied. The manufactures found were in the offset line selected based on the abstract content, hereafter the selected articles were evaluated in detail and based on relevance for this review and on the quality of the study, articles were included in this review. Studies on pregnancy immunology/embryology were non included.
3. The female reproductive cycles
In women, the reproductive cycle (menstrual cycle) is described co-ordinate to the gonadal activity or endometrial changes [nineteen]. In pigs, the reproductive cycle (estrous cycle) is classified by the sexual beliefs; estrus, where the grunter is sexually receptive, or non-estrus [20]. Both of the cycles can be described with two phases; the luteal and the follicular phase, separated by ovulation (Figure one).
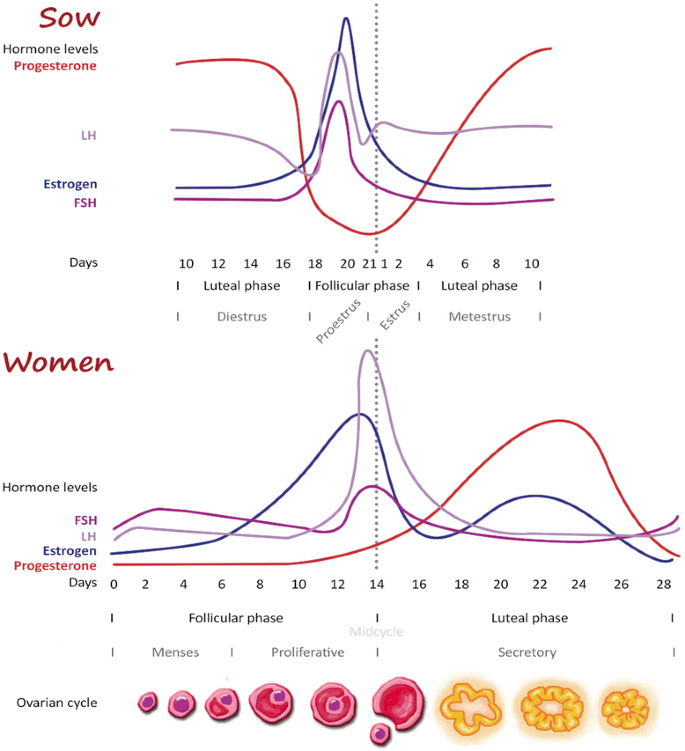
Comparison of the hormonal reproductive cycles in women and pigs. The estrous cycle in pigs begins and ends with ovulation/estrous [20,86]. The menstrual cycle in women begins and ends with the first of period, with the ovulation in the middle of the cycle [19]. Otherwise, the length of the cycle and the hormonal fluctuations are very like.
In the hog, a significant follicle growth occurs during the luteal phase (i.due east. the follicular phase overlaps the luteal phase), resulting in a slightly shorter cycle (nineteen–21 days) than in women, where the 2 phases are more than stringent separated and the cycle therefore lasts 28 days [19,twenty]. Even so, the mean length is very like between pigs and women.
The menses/menstruation, a encarmine uterine discharge, is specific for humans and some primates, unremarkably lasts 3–7 days and is related to the kickoff of the follicular phase [19]. Both women and pigs are spontaneous ovulators and continuously cycling [21]. A comparison of the changes in the reproductive hormones during the reproductive cycles is shown in Figure 1.
Both hormonal cycles are under control of the hypothalamic-pituitary-ovarian axis [19,20]. If no pregnancy occurs during an estrous wheel in the grunter, the non-meaning uterus secretes prostaglandin F2α (PGF-2α), which makes the corpus luteum regress (luteolysis) [20]. In women, the mechanism behind luteolysis is a bit more unclear, however, it is suggested that intraluteal PGF-2α plays a luteolysing role [22]. The important differences between the porcine estrous and human menstrual cycles are summarized in Table 1 together with the aforementioned parameters in primates and mice, to prove the level of similarity compared to these species.
4. The female genital tract in pigs and humans
4.1. Gross anatomy
The porcine uterus differs from the human by existence bicornuate [23] (Figure ii). The bicornuate elongation of the uterine body into two uterine horns creates a longer distance from the porcine cervix to the entrance of the Fallopian tubes than in women. In women the uterine body is approximately vii cm long [24] while in a one-year-old sexually mature Göttingen minipig gilt, each horn is an average 37.two ± 5.nine cm long (mean ± SD, northward = 12, unpublished data).
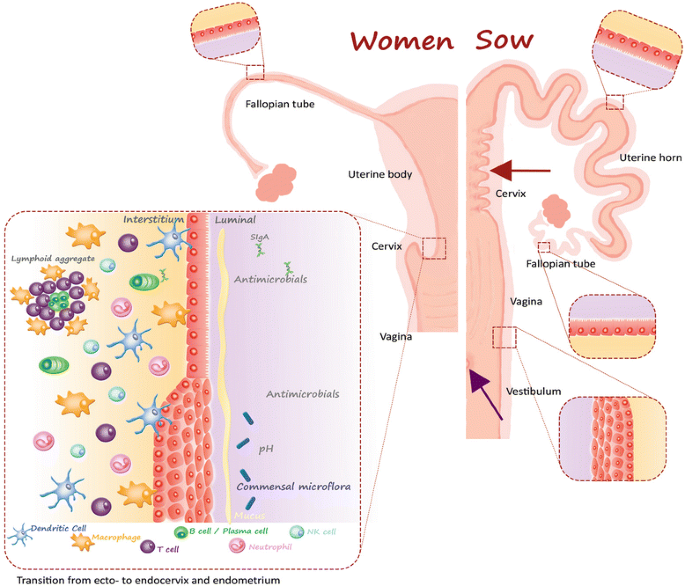
Comparison of the gross anatomy and epithelium in the genital tract in women and pigs. The porcine uterus differs macroscopically from the human simplex uterus by having bilateral horns (bicornuate) [23]. The porcine cervix displays a feature feature, non found in women; the cervical pulvini (red arrow) [23]. Furthermore, the porcine urethra opens on the ventral surface of the vagina (regal arrow) creating an urogenital sinus that opens to the outside through the common urogenital orifice [23]. In women, the urethra and vagina accept its ain separate openings to the outside [19]. Otherwise the porcine vagina is similar to the human one [92]. The homo cervix is divided into the ectocervix that protrudes into the vaginal canal and the endocervix, creating the cervical lumen. An example of the local allowed system in the female person genital tract is shown at the transition betwixt the ecto- and endocervix.
The cervix in Göttingen Minipigs is an average 7.5 ± 0.85 cm long, whereas the human cervix is effectually 2–iii cm [25]. The porcine cervix displays a characteristic feature, not found in women; the pulvini cervicales [23], which are a number of interdigitating prominent solid mucosal folds and protrusions throughout the length of the porcine neck. Furthermore, the porcine urethra opens on the ventral surface of the vagina, creating a urogenital sinus that opens to the exterior through the common urogenital orifice [11,14]. In women, the urethra and vagina have divide openings [19].
The vagina in women is approximately vii cm along the inductive curvature and 9 cm along the posterior curvature [25]. In Göttingen Minipigs the vagina is an average 13.viii ± 0.9 cm (mean ± SD, n = 12, unpublished data).
The Fallopian tubes are seven–14 cm long and 0.v–ane.2 cm in external diameter in women [26] and an average 17.3 ± 2.vii cm long and 0.4–0.v cm in diameter (mean ± SD, northward = 12, unpublished data) in 1-year-quondam Göttingen Minipigs.
4.2. Microscopic anatomy
Histology is a very of import tool in the evaluation of pathological changes in animal models. Therefore, it is of import to sympathise morphological differences between pigs and humans [12]. Generally, and common for both pigs and humans, the wall of the FGT consists of three layers: the mucosal, the muscular and the outer serosal layers [21]. The tunica mucosa facing the lumen (the endometrium), is built by the inner lamina epithelialis, lamina propria (connective tissue) and the tela submucosa. The muscular layer (tunica muscularis) is built by stratum circulare and stratum longitudinale. The outer tunica serosa (the perimetrium), facing the peritoneal and pelvic cavities, is congenital by a lamina propria and lamina epithelialis [21]. In the peritoneal crenel the lamina epithelialis of the tunica serosa has a simple squamous epithelium (visceral layer of the peritoneum) and in the pelvic crenel only loose connective tissue (adventitia) [21].
4.ii.ane. Vagina
The vagina is the entry site for most sexually transmitted diseases and therefore of bully importance when comparing the grunter model with humans [12]. The vaginal lamina epithelialis is made by non-keratinized stratified squamous epithelium and forms longitudinal folds called rugae in both women and pigs [12,27]. The porcine vaginal epithelium undergoes circadian alterations reaching a maximum thickness in the late proestrus [21]. The lamina propria consists of vascularized fairly dense connective tissue with no glands or mucosal muscular layer in both pigs and humans [21].
The vaginal mucosa is moisturized with secretions from the cervix. Cranially the porcine vagina is covered past a typical tunica serosa (i.e. loose connective tissue covered past the mesothelium) while caudally, a tunica adventitia, consisting of loose connective tissue is present. Both tunica serosa and adventitia incorporate large blood vessels, extensive venous and lymphatic plexuses and numerous nervus bundles and ganglia [21,28]. In women, the vagina is externally covered by adventitia, primarily built with rubberband fibers attaching the vagina to the surrounding connective tissues and organs [27]. Tunica muscularis is also like for pigs and humans with an inner layer of circularly arranged smooth muscle cells and an outer longitudinal layer, however the pig can take a thin layer within the round layer with longitudinally arranged fibers [21,27]. Studies have furthermore shown that the porcine vaginal permeability barrier, which is based on the lipid composition and intercellular lipid lamellae in the epithelium, closely resembles that of humans [12].
4.2.2. Cervix
The porcine neck has a thick, muscular wall rich in rubberband fibers [21,23], whereas the human but contains small-scale amounts of shine muscle and therefore mainly consists of dense connective tissue and elastic fibers [27].
The cervical lamina epithelialis differs between humans and pigs. In women the ectocervix has non-keratinized stratified squamous epithelium and the transformation zone separates information technology from the endocervix with a simple columnar epithelium [27]. In pigs, more than ninety% of the cervix may have a vaginal type of epithelium with stratified squamous epithelium that undergoes circadian alterations. The porcine cervical epithelium changes betwixt unproblematic columnar, pseudostratified and stratified squamous epithelium, with primarily columnar in diestrus and primarily stratified in rut [21].
Mutual for both species is the unproblematic columnar epithelium, which is mucinous with mucus secreting goblet cells. The corporeality of mucus secreted depends on the bicycle stage with an increased corporeality during estrus in pigs and midcycle in women (effectually ovulation). Much of the mucus passes to the vagina. Similarly the epithelium increase in thickness and edema develops during proestrus and estrus [21]. After ovulation the secretion decreases and the mucus becomes thicker [21].
4.2.three. Uterus
The human myometrium (tunica muscularis) is built by three muscular layers. The thick centre layer (stratum vasculare) contains many large vessels [27]. This highly vascularized and well-innervated stratum vasculare is, yet, indistinct in the squealer [21]. A tela submucosa, with dumbo irregular connective tissue, is not nowadays in the uterus in women, where the epithelium with lamina propria lie closely applied to the myometrium [27].
The epithelium is unproblematic columnar in both women and pigs, but in the squealer information technology increases significantly in height during heat and can plough into high pseudostratified columnar epithelium [21,29]. The endometrium and structure of the epithelial cells in women are likewise highly responsive to the hormonal changes and the thickness of the endometrium increases during the belatedly proliferative phase [21,xxx].
The endometrium in pigs and women can be characterized past ii zones or layers; the superficial functional layer (stratum functionale) and the deeper basal layer (stratum basale). The functional layer undergoes cyclic changes and degenerates partly or completely after pregnancy and estrus in the sus scrofa [21]. In humans, the degenerated tissue is shed during menstruation [27]. In contrast to women, the pigs' basal layer is more cellular and fibrous. It remains during all cyclic stages and is the source for restoration of the functional layer [21,27].
The uterine epithelium in pigs and women contains both ciliated cells and non-ciliated secretory cells [21] and branched and coiled (endometrial) glands that extend into the lamina propria [28]. In women, these glands are brusk and straight in the proliferative (follicular) phase and long and coiled in the secretory (luteal) phase [30]. In the porcine endometrium, growth and branching of the glands are stimulated by estrogen and the coiling and copious secretion by progesterone [21,29].
iv.two.four. Fallopian tubes
The Fallopian tubes are of special interest in genital Chlamydia research, as they represent the site of infection, where sterilizing pathology develops in women [31]. The mucosa at the Fallopian tubes is folded into longitudinal folds (plicae) and the epithelium has non-ciliated secretory cells and ciliated cells that aid in moving the sperm upwards and the ovum downwards. The mucosal plicae in the ampulla have secondary and sometimes tertiary folds creating a circuitous system of epithelial-lined spaces. The epithelial lining is made of a unmarried layer of columnar epithelial cells which sometimes is pseudostratified in pigs [21,32]. The epithelium undergoes circadian changes with the greatest height and ciliation in the belatedly follicular phase, and atrophy together with loss of cilia in the luteal stage [30].
The Fallopian tube in both pigs and humans can be separated into 3 parts; the isthmus, which is communicating with the uterus, the ampulla (the middle thin walled role), and the infundibulum that has fimbriae to catch the oocyte, when it is released into the peritoneal cavity during ovulation. The man Fallopian tubes furthermore have an extra compartment chosen the intramural part. Fertilization will accept place in the ampulla in both pigs (caudal ampulla) and humans [21,27].
iv.3. Anatomical and histological differences of relevance for a Chlamydia model
The slight anatomical differences in the pig are of import to consider when choosing the inoculation route and when evaluating the ascending capacity of an infection. The porcine cervical pulvini make the admission from the vagina to the uterus complicated in pigs and should be considered when choosing the inoculation method. Furthermore, the longer uterine body, in terms of uterine horns, is an important factor for the face validity of the pig model in evaluating ascending infections reaching the Fallopian tubes. In sexually young conventional pigs inoculation with C. trachomatis SvE resulted in an ascending infection with bacterial replication in the Fallopian tubes [sixteen].
A clear benefit of the porcine beefcake is the human-like prominent Fallopian tubes in the pig that potentially allows studying the tubal pathology induced by a C. trachomatis infection.
Since the columnar epithelial cells are the target cells for the C. trachomatis [xvi,33] it is important to be aware of the slightly different localization of the target cells. In women the columnar epithelial cells are found together with the transitional cells found in the endocervix and upper FGT [34]. In the grunter, the neck is dominated by stratified squamous epithelium and columnar cells are only consistently found in the porcine uterus [21,35], and therefore not at the vagino-cervical transition as in women. It is therefore recommended to inoculate pigs straight into the uterus.
five. Genetics
The majority of genes expressed in porcine female person reproductive tissues are expressed in homo FGT likewise [36]. As farther eluted to beneath, pigs share significantly more allowed-system related genes and proteins with humans than mice exercise [37].
six. The porcine immune arrangement compared to the human immune system
The porcine immune system is well characterized and highly resembles that of humans [11,36], although at that place are some differences. One of the differences is the anatomy of the lymph nodes, which are inverted in pigs [38]. The inverted lymph node structure only affects the lymphocyte migration through the lymph node. Porcine lymphocytes mainly get out the lymph node through high endothelial venules instead of efferent lymph vessels, as they do in humans [21,38,39]. Otherwise the physiology and immunologic reactions of the B and T cell areas in the lymph nodes do not differ [21,38].
About of the protein mediators of the immune system are present with the same structure and part in humans and pigs and most of the allowed cells identified in both species are similar [36,twoscore]. The distribution of leukocytes in the claret is very like in pigs and humans with a high per centum of neutrophils [41], nevertheless, inside the lymphocyte populations, pigs have a higher proportion of CD4+CD8+ double positive T cells and ƴδ T cells in the claret. Otherwise the distribution of the unlike lymphocyte populations in pigs and humans is quite like [eleven,36,forty,42] as summarized in Table 2.
The major histocompatibility complex (MHC) organisation in pigs, called the swine leukocyte antigen (SLA) system is very like to the human being leukocyte antigen system, in terms of polymorphic loci, haplotypes and differentiated expression on dissimilar jail cell populations [11,43]. Even so, resting porcine T lymphocytes can express MHCII before activation [11,43], whereas human T cells only express MHCII when activated [44].
All the cytokines in the human Th1/Th2/Th17/Treg paradigm have porcine orthologs [36], notwithstanding, it is suggested that IL-iv might play a different role in pigs [45]. The expression and frequency of immunoglobulins are quite similar (Table 2) except that IgD has not been demonstrated in pigs. Similar to humans, pigs have at least five IgG subclasses: IgG1, IgG2a, IgG2b, IgG3 and IgG4 [eleven]. Humans have 2 IgA heavy constant region genes (Cα) and therefore two subtypes of IgA designated IgA1 and IgA2 [46], whereas pigs merely have i Cα gene and therefore only i grade of IgA [46-48]. Circulating IgA is mostly bone marrow derived and monomeric in humans [49], while circulatory IgA in pigs is half dimeric IgA and half monomeric IgA [50]. The dimeric proportion of circulating IgA in the pig is, however, primarily derived from the intestinal synthesis and lymph. Due to the hepatic pIgR-mediated transcytosis of polymeric IgA (pIgA) to the bile, the dimeric IgA is thought to be relatively brusk-lived in the circulation [50]. The hepatic polymeric immunoglobulin receptor (pIgR)-mediated transcytosis of pIgA happens in both humans and pigs [50].
In women, IgA2 is known to be the predominant isotype bracket in the genital secretions [51] while this stardom cannot be made in the porcine FGT secretions.
When modeling genital infections and evaluating vaccine responses, the toll-like receptors (TLR) play a crucial part in recognition of the pathogens and induction of and decision-making/directing the allowed response. Information technology has been shown that the porcine TLR system is very similar to that of humans [41]. In terms of cytokines such as the neutrophil chemokine IL-8, the coding factor carried by humans and pigs is an ortholog [41]. Furthermore, human- and porcine macrophages produce indoleamine 2,3-dioxygenase (IDO) in response to lipopolysaccharide (LPS) and Interferon gamma (IFN-ɣ) stimulation [36,41].
6.ane. The genital mucosal immune response
The genital mucosal immune responses are of specific importance when using the pig as a model of human being genital C. trachomatis infections. The genital immune response is challenged in the sense that information technology has to tolerate sperm, the semi-allogeneic conceptus and the commensal vaginal flora, while it must mount defense responses against sexually transmitted pathogens in society to eliminate them [52].
The genital immune system consists of both innate and adaptive factors. The innate system is primarily built by the epithelial barrier, the product of antimicrobial agents and cytokines past the epithelial cells and the innate allowed cells [xl,53]. Both innate and adaptive humoral mediators and immune cells in the genital immune system are regulated by progesterone and estradiol and therefore fluctuate through the menstrual or estrous cycles [53].
The epithelial cells in the FGT with interconnecting tight junctions play an important office in the immune protection by providing a strong physical barrier, transporting antibodies to the mucosal surface, secreting antibacterial compounds and by recruiting immune cells [54,55]. The sex hormones regulate the structural changes in the epithelium during the cycle. Under the influence of estrogen, the integrity and strength of tight junctions in the epithelial barrier, is significantly weakened in women [54,56]. The secretion of antimicrobial compounds is besides suppressed during the midcycle in women [53,57].
To preserve an intact protective bulwark, the genital mucosal immune response is often non-inflammatory to avoid inflammation-mediated injuries usually caused by phagocytic action and complement activation [55]. Well-nigh of the antigens in the FGT are therefore met with mucosal tolerance [55].
6.1.1. Distribution of immune cells in the genital tract tissue
The genital mucosa does non accept immune anterior sites such as the nasal-associated lymphoid tissue or intestinal Peyer's patches [55]. Thus, the genital mucosa lacks an organized center to disseminate antigen-stimulated B and T lymphocytes to the distinct sites of the mucosa. Even so, lymphoid aggregates (LA) are present in the female genital mucosa of both pigs [35] and humans [55] and leukocytes are dispersed throughout the mucosa of the FGT [58] as illustrated in Effigy 2.
The LA are located in the basal layer of the endometrium shut to the base of the uterine epithelial glands and congenital by a cadre of B cells surrounded by T cells and an outer layer of macrophages [58]. The T cells in the LA are primarily CD8+ T cells, however, CD4+ T cells are also nowadays in variable numbers in the LA [58]. Both CD4+ and CD8+ T cells are found every bit intraepithelial lymphocytes and dispersed throughout the subepithelial tissue [58]. Aggregates of NK cells tin also be found in the endometrium merely they are placed in close contact with the luminal epithelium [58].
The leukocytes present in the FGT covers macrophages, dendritic cells, NK cells, neutrophils, B cells and T cells [53,59,60] with lymphocytes being the predominant allowed jail cell type in both pigs and women [35,61,62]. The number of immune cells and the size of LA are under stiff hormonal influence and fluctuate through the cycle [55,58] as summarized in Table iii.
6.1.2. The humoral genital allowed response
The immunoglobulins found in the FGT either have been locally produced by subepithelial plasma cells, or derived from the apportionment [63]. Although IgG producing plasma cells can be found in the FGT [64], genital IgG is mainly derived from the apportionment [63,65-67] and transported to the mucosal surface past mechanisms such as passive leakage, paracellular improvidence or receptor-mediated transport [63,65]. In dissimilarity, genital IgM and IgA are primarily derived from the subepithelial plasma cells [65,68-70] with up to 95% of the porcine IgA existence locally produced [71] and up to 70% of the IgA existence locally produced in women [55]. When produced locally, the polymeric secretory IgA (sIgA) is actively transported across the mucosal epithelia cells by the polymeric immunoglobulin receptor (pIgR) [65,66]. The secretion of sIgA primarily takes place in the cervix due to the focused pIgR localization in the neck in women [72]. The pIgR is besides expressed in the uterus, but to a bottom extent and in variable levels due to hormonal regulation [55].
Usually, sIgA is the predominant isotype found in mucosal secretions, such as the intestinal fluid. However, in the secretions from the FGT, there is a greater proportion of IgG compared to sIgA [65,73-75].
The FGT humoral immune response is under strong hormonal influence during the menstrual or estrous cycle [57,74]. The cyclic fluctuations in the antibody levels are compared in Table 3. The data on cycle-dependent variations in the level of antibodies in pigs is sparse and more knowledge is needed within this area.
6.one.iii. Immunological differences of relevance for a Chlamydia model
The nearly important immunological difference with potential influence on Chlamydia models is the slightly different influx of allowed cells in the porcine FGT, characterized by an increase in neutrophils during heat. It should exist kept in mind that this increased innate response during estrus could influence the establishment of infection.
seven. The vaginal flora and pH
In women, the vaginal microflora is known to play an important office in the innate genital immune arrangement past inhibiting the colonization of pathogens [76,77]. Lactobacilli and other lactic acid producing bacteria are particularly associated with equilibrium in the vaginal flora and inhibition of the growth of pathogens [76,78,79].
16S rRNA gene sequencing has allowed a thorough identification of the vaginal flora in women and the about common bacteria are: Lactobacillus spp., Staphylococcus spp., Ureaplasma urealyticum, Corynebacterium vaginale, Streptococcus spp., Peptostreptococcus spp., Gardnerella vaginalis, Bacteroides spp., Mycoplasma spp., Enterococcus spp., Escherichia coli, Veillonella spp., Bifidobacterium spp. and Candida spp.. However, the species limerick can be very different betwixt individuals and during the menstrual cycle [52,76,79]. In women, the lactic acid producing bacteria play an important role by contributing to an acidic environment with a pH of 3.five–5 [52].
In healthy pigs the vaginal flora has been characterized by culture dependent methods and was found to include both aerobic and anaerobic leaner with the well-nigh prominent existence the following: Streptococcus spp., E. coli, Staphylococcus spp., Corynebacterium spp., Micrococcus spp. and Actinobacillus spp. [80]. Based on our genetic screening of vaginal swabs from Göttingen Minipigs, it is evident that the above mentioned bacteria are present, just not dominating. Streptococcus spp. constituted on average one.iv% on the vaginal flora, East. coli 3.seven%, and Staphylococcus 0.iv%. Furthermore, we found that the vaginal flora was not dominated by lactobacillus as in humans. Lactobacillaceae constituted on boilerplate 3.9% of the total vaginal flora in Göttingen Minipigs. The vaginal flora in Göttingen Minipigs seemed to exist dominated by the following: unclassified genera belonging to Gammaproteobacteria, unclassified genera from Clostridiales, Yersinia, Paenibacillus, Listeria, Syntrophus, Heliobacterium, Faecalibacterium, Kineococcus and Proteus (unpublished data).
An old study showed that the FGT mean pH in estrus in pigs is seven.02 in the oviduct, 6.98 in the uterus, vii.49 in the cervix and half dozen.61 in the vagina [81]. Our own data, based on vaginal pH measurements with a pH electrode (Mettler-Toledo InLab® Surface Electrode, Sigma-Aldrich Broendby, Denmark), confirmed that the vaginal pH is just effectually neutral (~seven) in both prepubertal and sexually mature Göttingen Minipigs.
8. Important differences betwixt rodents and minipigs
The primary aim of this review was to compare the female reproductive physiology of humans and pigs, however, every bit a concluding department, we found it important to highlight where the minipig shows significant differences to the commonly used murine model in Chlamydia research. Similar comparisons of humans and mice has been done elsewhere [4,82,83], and just primary points will be included here.
The reproductive cycle is significantly shorter in mice, having a 4–5 day bicycle due to the lack of progesterone-producing corpora lutea and thereby a luteal phase, if no coital stimulation occurs [84]. Anatomically, the murine uterus is bicornuate and much smaller than the porcine and human ones [83]. Histologically, the vagina displays keratinized squamous epithelium during rut, whereas porcine and human epithelium does not keratinize [83].
Inside the immune system, the composition of circulating leukocytes is significantly different with a lower percentage of neutrophils and a corresponding college abundance of lymphocytes in mice compared to pigs and humans [82]. Furthermore, and chiefly for the Chlamydia model, murine macrophages do not produce IDO in response to LPS and IFN-ɣ stimulation, by contrast humans and porcines do [36,41]. Furthermore, murine macrophages produce nitric oxide (NO) in response to stimulation with LPS, whereas human and porcine macrophages exercise not [36]. There is besides a great divergence in the expression of cytokines such as IL-viii, a potent neutrophil chemokine expressed in pigs and humans, but not in mice. In mice keratinocyte-derived chemokine and macrophage inflammatory protein-ii are considered to be the IL-8 counterpart [41].
In the FGT, the influx of immune cells happens slightly differently in mice, compared to pigs and humans. In the murine endometrium an influx of leukocytes is seen in the proestrus, during oestrus the leukocytes are most absent, during metestrus they are prominent and during diestrus an infiltration is seen [83]. The fluctuations in antibody levels in the murine FGT shows a similar pattern for IgG, with a lower level during estrus, while for IgA, information technology is opposite that of pigs and women, with mice having a higher level during estrus [85].
9. Conclusions
This comparison of the porcine and human FGT reveals clear similarities and gives an understanding of the differences between the species. Despite the bicornuate porcine uterus with a urogenital sinus and cervical pulvini, the anatomical and morphological construction and proportion of layers with cyclic alterations is very similar in humans and pigs. The hormonal cycles are closely related, only differing slightly in cycle duration, and origin of luteolysing hormone. The general immune arrangement and the immune system associated with the FGT show swell similarities. The antibody levels on the genital mucosa shows like cyclic fluctuations in pigs and women, but the allowed cell infiltration in the genital mucosa differs slightly between women and pigs, namely in the influx of neutrophils in the porcine endometrium during estrus. The porcine vaginal flora differs from the man by not being dominated by lactobacilli and the vaginal pH is slightly higher in pigs than in women.
Information technology is difficult to tell the verbal significance of the differences and similarities between the FGT in women and pigs and interpretation of data from brute models should always exist done with caution. The similarities plant in this review, however, suggest that the pig adds a greater predictive value to FGT studies than what can be accomplished past studies in rodent models. Non-human primates is the species nearly closely related to humans, but ethical concerns and the relative ease of working with pigs advise the pig to be an advantageous model of human reproductive disorders such equally C. trachomatis infection.
Abbreviations
- APC:
-
Antigen presenting prison cell
- FGT:
-
Female genital tract
- IDO:
-
Indoleamine two,3-dioxygenase
- IFN-ɣ:
-
Interferon gamma
- Ig:
-
Immunoglobulin
- LA:
-
Lymphoid aggregates
- LGT:
-
Lower genital tract
- LPS:
-
Lipopolysaccharide
- MHC:
-
Major Histocompatibility complex
- NHP:
-
Non-human primates
- NO:
-
Nitric Oxide
- PGF-2α:
-
Prostaglandin-F2α
- pIgR:
-
Polymeric immunoglobulin receptor
- pIgA:
-
Polymeric immunoglobulin A
- sIgA:
-
Secretory Immunoglobulin A
- SLA:
-
Swine leukocyte antigen
- TLR:
-
Toll-like receptor
- UGT:
-
Upper genital tract
14. References
-
Lantier F (2014) Animal models of emerging diseases: An essential prerequisite for research and development of control measures. Anim Front 4:vii–12
-
De Clercq E, Kalmar I, Vanrompay D (2013) Fauna models for studying female genital tract infection with Chlamydia trachomatis. Infect Immun 81:3060–3067
-
O'Meara CP, Andrew DW, Beagley KW (2014) The mouse model of Chlamydia genital tract infection: A review of infection, disease, immunity and vaccine development. Curr Mol Med 14:396–421
-
Mestas J, Hughes CCW (2004) Of mice and non men: differences between mouse and human immunology. J Immunol 172:2731–2738
-
Schautteet Yard, Stuyven E, Beeckman DSA, Van Acker S, Carlon 1000, Chiers K, Cox E, Vanrompay D (2011) Protection of pigs against Chlamydia trachomatis claiming by administration of a MOMP-based DNA vaccine in the vaginal mucosa. Vaccine 29:1399–1407
-
Denayer T, Stöhr T, Van Roy One thousand (2014) Creature models in translational medicine: Validation and prediction. New Horizons Transl Med ii:five–11
-
Hein WR, Griebel PJ (2003) A road less travelled : large creature models in immunological research. Nat Rev Immunol 3:79–85
-
Girard MP, Plotkin SA (2012) HIV vaccine development at the turn of the 21st century. Curr Opin HIV AIDS 7:4–9
-
Schautteet K, Stuyven E, Cox E, Vanrompay D (2011) Validation of the Chlamydia trachomatis genital challenge grunter model for testing recombinant protein vaccines. J Med Microbiol 60:117–127
-
Dodet B (2014) Current barriers, challenges and opportunities for the development of effective STI vaccines: Signal of view of vaccine producers, biotech companies and funding agencies. Vaccine 32:1624–1629
-
Bode G, Clausing P, Gervais F, Loegsted J, Luft J, Nogues Five, Sims J (2010) The utility of the minipig as an brute model in regulatory toxicology. J Pharmacol Toxicol Methods 62:196–220
-
Squier CA, Mantz MJ, Schlievert PM, Davis CC (2008) Porcine vagina ex vivo as a model for studying permeability and pathogenesis in mucosa. J Pharm Sci 97:9–21
-
Turk JR, Henderson KK, Vanvickle GD, Watkins J, Laughlin MH (2005) Arterial endothelial function in a porcine model of early stage atherosclerotic vascular illness. Int J Exp Pathol 86:335–345
-
Swindle MM (2007) Swine in the Laboratory. CRC Printing, Boca Raton
-
The Göttingen minipig [www.minipigs.dk] Accessed 5 May 2015
-
Vanrompay D, Hoang TQT, De Vos 50, Verminnen 1000, Harkinezhad T, Chiers K, Morré SA, Cox E (2005) Specific-pathogen-gratis pigs as an animal model for studying chlamydia trachomatis genital infection. Infect Immun 73:8317–8321
-
The PubMed Database [http://www.ncbi.nlm.nih.gov/pubmed/] Accessed 20 Oct 2014
-
Google Scholar Database [https://scholar.google.dk/] Accessed 20 October 2014
-
Silverthorn DU (2007) Human Physiology. Pearson Benjamin Cummings, United States of America
-
Senger PL (2005) Pathways to Pregnancy and Parturition. Current Conceptions Inc., Washington
-
Eurell JA, Frappier BL (2006) Dellmann's Textbook of Veterinarian Histology. Blackwell Publishing, Us of America
-
Corpus Luteum [http://world wide web.glowm.com/section_view/heading/Corpus Luteum/item/290] Accessed 5 May 2015
-
König HE, Liebich HG (2009) Anatomie Der Haussäugetiere. Schattauer, Stuttgart
-
Konar H (2014) DC Dutta's Textbook of Obstetrics. Jaypee Brothers Medical Publishers Ltd., New Delhi
-
Konar H (2014) DC Dutta'southward Textbook of Gynecology. Jaypee Brothers Medical Publishers Ltd, New Delhi
-
Ledger WL, Tan SL, Bahathiq AOS (2010) The Fallopian Tube in Infertility and IVF Practice. Cambridge University Press, Cambridge
-
Krause WJ (2005) Krause's Essential Man Histology For Medical Students. Universal Publishers, United States of America
-
Bacha WJ, Bacha LM (2000) Colour Atlas of Veterinary Histology. Lippincott Williams & Wilkins, Us of America
-
Kaeoket K, Persson E, Dalin A-1000 (2002) Blunder to "The sow endometrium at different stages of the estrus wheel: studies on morphological changes and infiltration by cells of the immune organisation" [Anim. Reprod. Sci. 65 (2001) 95–114]. Anim Reprod Sci 73:89–107
-
Strauss JF, Barbieri RL (2014) Yen and Jaffe'south Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Saunders Elsevier, Philadelphia
-
Darville T, Hiltke TJJ (2010) Pathogenesis of genital tract disease due to Chlamydia trachomatis. J Infect Dis 201(Suppl 2):114–125
-
Hussein AM, Newby TJ, Bourne FJ (1983) Immunohistochemical studies of the local allowed arrangement in the reproductive tract of the sow. J Reprod Immunol v:1–xv
-
Brunham RC, Rey-Ladino J (2005) Immunology of Chlamydia infection: implications for a Chlamydia trachomatis vaccine. Nat Rev Immunol 5:149–161
-
Howard C, Friedman DL, Leete JK, Christensen ML (1991) Correlation of the percent of positive Chlamydia trachomatis direct fluorescent antibody detection tests with the adequacy of specimen collection. Diagn Microbiol Infect Dis xiv:233–237
-
The porcine cervix [http://ex-epsilon.slu.se:8080/archive/00003222/01/EEF_Karin_Edstrom.pdf] Accessed May 6, 2015
-
Meurens F, Summerfield A, Nauwynck H, Saif 50, Gerdts V (2012) The squealer: a model for human infectious diseases. Trends Microbiol 20:50–57
-
McAnulty PA, Dayan Advertisement, Ganderup Due north-C, Hastings KL (eds) (2011) The Minipig in Biomedical Research. CRC Press, United States of America
-
Binns RM, Pabst R (1994) Lymphoid tissue structure and lymphocyte trafficking in the pig. Vet Immunol Immunopathol 43:79–87
-
Rothkötter H-J (2009) Anatomical particularities of the porcine allowed system--a dr.'south view. Dev Comp Immunol 33:267–272
-
Mair KH, Sedlak C, Käser T, Pasternak A, Levast B, Gerner W, Saalmüller A, Summerfield A, Gerdts Five, Wilson HL, Meurens F (2014) The porcine innate immune organisation: An update. Dev Comp Immunol 45:321–343
-
Fairbairn Fifty, Kapetanovic R, Sester DP, Hume DA (2011) The mononuclear phagocyte organisation of the pig equally a model for understanding human innate immunity and disease. J Leukoc Biol 89:855–871
-
Zuckermann FA, Gaskins HR (1996) Distribution of porcine CD4 / CD8 double-positive T lymphocytes in mucosa-associated lymphoid tissues. Immunology 87:493–499
-
Saalmüller A, Maurer S (1994) Major histocompatibility antigen grade 2 expressing resting porcine T lymphocytes are potent antigen-presenting cells in mixed leukocyte culture. Immunobiology 190:23–34
-
Holling TM, Schooten E, van Den Elsen PJ (2004) Function and regulation of MHC class 2 molecules in T-lymphocytes: of mice and men. Hum Immunol 65:282–290
-
Murtaugh MP, Johnson CR, Xiao Z, Scamurra RW, Zhou Y (2009) Species specialization in cytokine biology: Is interleukin-4 central to the TH1-TH2 prototype in swine? Dev Comp Immunol 33:344–352
-
Snoeck 5, Peters IR, Cox E (2006) The IgA system: a comparison of structure and role in dissimilar species. Vet Res 37:455–467
-
Gibbons DL, Spencer J (2011) Mouse and human being intestinal amnesty: aforementioned ballpark, different players; different rules, same score. Mucosal Imunol 4:148–157
-
Mills FC, Harindranath N, Mitchell M, Max EE (1997) Enhancer complexes located downstream of both homo immunoglobulin Calpha genes. J Exp Med 186:845–858
-
Van der Boog PJM, van Kooten C, de Fijter JW, Daha MR (2005) Function of macromolecular IgA in IgA nephropathy. Kidney Int 67:813–821
-
Vaerman J, Langendries A, Reinhardt P, Rothkötter H (1997) Contribution of serum IgA to intestinal lymph IgA, and vice versa, in minipigs. Vet Immunol Immunopathol 58:301–308
-
Cerutti A (2008) The regulation of IgA course switching. Nat Rev Immunol eight:421–434
-
Quayle AJ (2002) The innate and early immune response to pathogen challenge in the female person genital tract and the pivotal role of epithelial cells. J Reprod Immunol 57:61–79
-
Hickey DK, Patel MV, Fahey JV, Wira CR (2011) Innate and adaptive immunity at mucosal surfaces of the female reproductive tract: stratification and integration of immune protection against the transmission of sexually transmitted infections. J Reprod Immunol 88:185–194
-
Ochiel DO, Fahey JV, Ghosh M, Haddad SN, Wira CR (2008) Innate immunity in the female reproductive tract: Role of sexual practice hormones in regulating uterine epithelial cell protection against pathogens. Curr Women's Heal Rev 4:102–117
-
Russell MW, Mestecky J (2002) Humoral immune responses to microbial infections in the genital tract. Microbes Infect iv:667–677
-
Wira CR, Fahey JV, Ghosh M, Patel MV, Hickey DK, Ochiel Do (2010) Sex hormone regulation of innate immunity in the female reproductive tract: the office of epithelial cells in balancing reproductive potential with protection against sexually transmitted pathogens. Am J Reprod Immunol 63:544–565
-
Stanberry LR, Rosenthal SL (Eds) (2012) Sexually Transmitted Diseases: Vaccines, Prevention, and Command. Academic Press, Oxford.
-
Yeaman GR, Wirat R, Guyre PM, Gonzalez J, Collins JE, Stern JE (1997) Unique CD8 T jail cell-rich lymphoid aggregates in homo uterine endometrium. J Leukoc Biol 61:427–435
-
Booker SS, Jayanetti C, Karalak S, Hsiu J-G, Archer DF (1994) The outcome of progesterone on the aggregating of leukocytes in the human endometrium. Am J Obstet Gynecol 171:139–142
-
Kamat BR, Isaacson PG (1987) Immmunocytochemical distribution of leukocytic subpopulations in human endometrium. Am J Pathol 127:66–73
-
Dalin A-M, Kaeoket M, Persson E (2004) Immune prison cell infiltration of normal and dumb sow endometrium. Anim Reprod Sci 82–83:401–413
-
Bischof RJ, Brandon MR, Lee C-S (1994) Studies on the distribution of immune cells in the uteri of prepubertal and cycling gilts. J Reprod Immunol 26:111–129
-
Mestecky J, Alexander RC, Wei Q, Moldoveanu Z (2011) Methods for evaluation of humoral immune responses in human genital tract secretions. Am J Reprod Immunol 65:361–367
-
Rebello R, Green F (1975) A study of secretory immune system in the female reproductive tract. Br J Obstet Gynaecol 82:812–816
-
Wright PF (2011) Inductive/effector mechanisms for humoral immunity at mucosal sites. Am J Reprod Immunol 65:248–252
-
Kutteh WH, Prince SJ, Hammond KR, Kutteh CC, Mestecky J (1996) Variations in immunoglobulins and IgA subclasses of human uterine cervical secretions around the time of ovulation. Clin Exp Immunol 104:538–542
-
Hussein AM, Newby TJ, Stokes CR, Bourne FJ (1983) Quantitation and origin of immunoglobulins A, One thousand and Thou in the secretions and fluids of the reproductive tract of the sow. J Reprod Immunol five:17–26
-
Woof JM, Mestecky J (2005) Mucosal immunoglobulins. Immunol Rev 206:64–82
-
Kutteh WH, Prince SJ, Mestecky J (1982) Tissue origins of human polymeric and monomeric IgA. J Immunol 128:990–995
-
Irish potato M (2011) Janeway's Immunobiology. Garland Science, United states of America
-
Butler JE, Chocolate-brown WR (1994) The immunoglobulins and immunoglobulin genes of swine. Vet Immunol Immunopathol 43:5–12
-
Iwasaki A (2010) Antiviral immune responses in the genital tract: clues for vaccines. Nat Rev Immunol x:699–711
-
Naz RK (2012) Female person genital tract immunity: distinct immunological challenges for vaccine evolution. J Reprod Immunol 93:1–8
-
Mestecky J, Raska G, Novak J, Alexander RC, Moldoveanu Z (2010) Antibody-mediated protection and the mucosal immune system of the genital tract: relevance to vaccine blueprint. J Reprod Immunol 85:81–85
-
Hafner LM, Wilson DP, Timms P (2014) Evolution status and future prospects for a vaccine against Chlamydia trachomatis infection. Vaccine 32:1563–1571
-
Farage MA, Miller KW, Sobel JD (2010) Dynamics of the vaginal ecosystem - hormonal influences. Infect Dis Res Treat iii:1–xv
-
Zhou X, Bent SJ, Schneider MG, Davis CC, Islam MR, Forney LJ (2004) Label of vaginal microbial communities in adult good for you women using cultivation-contained methods. Microbiology 150:2565–2573
-
Mastromarino P, Di Pietro Thousand, Schiavoni Thousand, Nardis C, Gentile M, Sessa R (2014) Effects of vaginal lactobacilli in Chlamydia trachomatis infection. Int J Med Microbiol 304:654–661
-
Larsen B, Monif GRG (2001) Understanding the bacterial flora of the female person genital tract. Clin Infect Dis 32:69–77
-
Bara Grand, McGowan M, O'Boyle D, Cameron R (1993) A study of the microbial flora of the inductive vagina of normal sows during different stages of the reproductive cycle. Aust Vet J 70:256–259
-
Mather EC, Day BN (1977) "IN VIVO" pH values of the estrous reproductive tract of the gilt. Theriogenology 8:323–327
-
Haley PJ (2003) Species differences in the structure and function of the immune system. Toxicology 188:49–71
-
Treuting PM, Dintzis SM (eds) (2012) Comparative Beefcake and Histology - a Mouse and Human Atlas. Academic, Oxford
-
Goldman JM, Murr Every bit, Cooper RL (2007) The rodent estrous cycle: Characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res eighty:84–97
-
Gallichan WS, Rosenthal KL (1996) Effects of the estrous cycle on local humoral immune responses and protection of intranasally immunized female person mice confronting herpes simplex virus type 2 infection in the genital tract. Virology 224:487–497
-
Manipulation of the estrous bicycle in swine [http://www2.ca.uky.edu/agc/pubs/asc/asc152/asc152.htm] Accessed May half dozen, 2015
-
Prostaglandins and the reproductive wheel [http://www.glowm.com/section_view/heading/Prostaglandins and the Reproductive Cycle/item/313] Accessed May five, 2015
-
D'Hooghe TM, Nyachieo A, Chai DC, Kyama CM, Spiessens C, Mwenda JM (2008) Reproductive research in non-human primates at Institute of Primate Inquiry in Nairobi, Kenya (WHO Collaborating Center): a platform for the development of clinical infertility services? Hum Reprod 2008:102–107
-
Wolfe-Coote S (ed) (2005) The Laboratory Primate. Elsevier Bookish Printing, San Diego
-
Swindle MM, Makin A, Herron AJ, Clubb FJ, Frazier KS (2012) Swine as models in biomedical enquiry and toxicology testing. Vet Pathol 49:344–356
-
Coleman DL, Kaliss N, Dagg CP, Russell ES, Fuller JL, Staats J, Green MC, Russell CPDES, Staats JLFJ (1966) Biology of the Laboratory Mouse. Dover Publications inc., New York
-
D'Cruz OJ, Erbeck D, Uckun FM (2005) A study of the potential of the pig as a model for the vaginal irritancy of benzalkonium chloride in comparison to the nonirritant microbicide PHI-443 and the spermicide vanadocene dithiocarbamate. Toxicol Pathol 33:465–476
-
Berrington JE, Barge D, Fenton AC, Cant AJ, Spickett GP (2005) Lymphocyte subsets in term and significantly preterm Britain infants in the first yr of life analysed by single platform flow cytometry. Clin Exp Immunol 140:289–292
-
Pomorska-Mól M, Markowska-Daniel I (2011) Age-dependent changes in relative and absolute size of lymphocyte subsets in the claret of pigs from nascence to slaughter. Bull Vet Inst Pulawy 55:305–310
-
Pudney J, Quayle AJ, Anderson DJ (2005) Immunological microenvironments in the homo vagina and cervix: Mediators of cellular immunity are full-bodied in the cervical transformation zone. Biol Reprod 73:1253–1263
-
White Hard disk, Crassi KM, Givan A, Stern JE, Memoli VA, Green WR, Wirat CR (1997) CD3 + CD8+ CTL activity within the homo female reproductive tract. J Immunol 158:3017–3027
-
Jiwakanon J, Persson Due east, Kaeoket K, Dalin A-K (2005) The sow endosalpinx at unlike stages of the oestrous wheel and at anoestrus: Studies on morphological changes and infiltration past cells of the immune system. Reprod Domest Anim 40:28–39
-
Usala S, Usala F, Holt J, Schumacher G (1989) IgG and IgA content of vaginal fluid during the menstrual cycle. J Reprod Med 34:292–294
-
Nardelli-Haefliger D, Wirthner D, Schiller JT, Lowy DR, Hildesheim A, Ponci F, De Grandi P (2003) Specific antibody levels at the neck during the menstrual cycle of women vaccinated with human being papillomavirus 16 virus-like particles. J Natl Cancer Inst 95:1128–1137
-
Keller MJ, Guzman East, Hazrati E, Kasowitz A, Cheshenko N, Wallenstein South, Cole AL, Cole AM, Profy AT, Wira CR, Hogarty M, Herold BC (2007) PRO 2000 elicits a turn down in genital tract immune mediators without compromising intrinsic antimicrobial activity. AIDS 21:467–476
-
Thiruchelvam U, Dransfield I, Saunders PTK, Critchley HOD (2013) The importance of the macrophage within the human endometrium. J Leukoc Biol 93:217–225
-
Yeaman GR, Collins JE, Fanger MW, Gratuitous R (2001) CD8 + T cells in homo uterine endometrial lymphoid aggregates : evidence for accumulation of cells by trafficking. Immunology 102:434–440
-
Shaw JLV, Fitch P, Cartwright J, Entrican Thou, Schwarze J, Critchley HOD, Hornea AW (2011) Lymphoid and myeloid prison cell populations in the non-meaning human Fallopian tube and in ectopic pregnancy. J Reprod Immunol 89:84–91
-
Kaeoket K, Dalin A-One thousand, Magnusson U, Persson Due east (2002) Corrigendum to "The sow endometrium at different stages of the oestrous wheel: studies on the distribution of CD2, CD4, CD8 and MHC class Two expressing" cells. [Anim. Reprod. Sci. 68 (2001) 99-109]. Anim Reprod Sci 73:109–119
Writer data
Authors and Affiliations
Corresponding author
Boosted information
xi. Competing interests
The authors declare that they have no competing interests.
12. Authors' contributions
EL performed the literature study, drafted the structural design of the review and was responsible for writing the manuscript. FF, GJ and JSA contributed intellectually with a critical revision of the manuscript. All authors accept read and approved the final manuscript.
xiii. Authors' information
EL is DVM and currently a PhD educatee at University of Copenhagen and Statens Serum Institut, Denmark. For two years, EL has been working on a project, focusing on the development of a minipig model for human genital Chlamydia, for evaluation of vaccine candidates. FF is the Caput of Chlamydia Vaccine Research at Statens Serum Institut, Denmark. FF is responsible for pre-clinical antigen discovery, vaccine design and formulation. GJ is professor in Immunology and Vaccinology at the National Veterinarian Institute with special expertise in porcine and bovine immune responses and immunological correlates of vaccine mediated protection. JSA is professor in Veterinary Reproduction and Obstetrics with a PhD in pathology. JSA has studied genital tract inflammation for several years and has supervised the evolution of a porcine model for genital Chlamydia in women since 2010.
Rights and permissions
Open up Access This commodity is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/past/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided yous give appropriate credit to the original writer(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The Creative Commons Public Domain Dedication waiver (http://creativecommons.org/publicdomain/zero/i.0/) applies to the data made available in this article, unless otherwise stated.
Reprints and Permissions
Well-nigh this article
Cite this article
Lorenzen, E., Follmann, F., Jungersen, G. et al. A review of the human vs. porcine female person genital tract and associated immune system in the perspective of using minipigs every bit a model of human being genital Chlamydia infection. Vet Res 46, 116 (2015). https://doi.org/10.1186/s13567-015-0241-ix
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/s13567-015-0241-9
Keywords
- Fallopian Tube
- Female Genital Tract
- Lymphoid Amass
- Vaginal Flora
- Swine Leukocyte Antigen
Source: https://veterinaryresearch.biomedcentral.com/articles/10.1186/s13567-015-0241-9
Posted by: hallwhats1993.blogspot.com

0 Response to "Which Animal Has A Vagina Most Like A Human"
Post a Comment